Modeling Defects in Materials and Quantum Dots
The simulation of defects in materials is a crucial area of research with wide-ranging implications for both fundamental science and practical applications. Understanding how atomic-scale defects influence the properties of materials can lead to advancements in technologies such as photovoltaics, photocatalysis, and sensor devices. By leveraging computational chemistry techniques to model and predict the behavior of defects, researchers can gain deeper insights into material properties, optimize manufacturing processes, and develop novel materials with tailored functionalities.
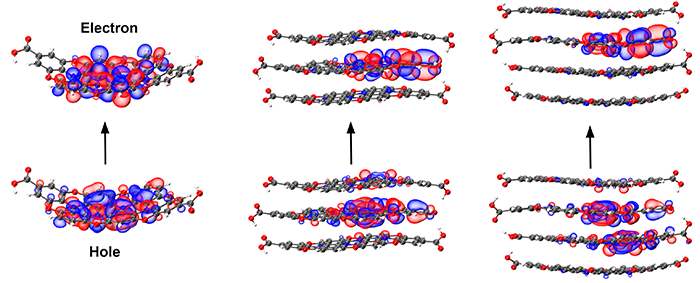
One of my notable projects involves investigating the emission properties of bottom-up synthesized carbon dots (CDs). CDs, produced via solvothermal methods using small organic molecules, are known for their cost-effective production, biocompatibility, strong light absorption, and versatile fluorescence. In collaboration with the Gruebele and Link groups at the University of Illinois Urbana-Champaign (UIUC), we explored how the atomic structure of CDs synthesized from different benzene diamine (DAB) isomers influences their emission properties. Using linear response TDDFT calculations on extensive datasets of carbon dot structures, we discovered that sp3 nitrogen defects in ortho and para CDs disrupt conjugation, resulting in smaller emissive domains (see illustration below). In contrast, meta DAB-derived CDs exhibit variably sized conjugated domains, leading to emissions strongly dependent on domain size variability. Our findings highlight the significant impact of precursor isomer selection on the emission characteristics of CDs, providing valuable insights for their application in optoelectronic devices. (See my paper for further details.)
Another key area of our research focuses on quantifying hot-electron transfer through plasmon-mediated pathways. Understanding hot carrier dynamics and charge transfer processes within metal-semiconductor heterostructures is pivotal for enhancing the efficiency of photovoltaic and photocatalytic devices. In collaboration with the Link group at UIUC, we aimed to quantify the electron transfer efficiency in AuNR@TiO2 core-shell heterostructures, distinguishing between direct and indirect charge transfer mechanisms. I developed an open-source software package to compute the charge-transfer component, validating the chemical interface damping (CID) model proposed by Persson. Our results showed that the experimentally observed broadening of localized surface plasmon resonance (LSPR) is primarily due to direct charge transfer across the interface. This work significantly advances our understanding of charge injection in metal-semiconductor heterostructures, indicating that direct plasmon-induced charge transfer holds promise for enhancing hot carrier extraction efficiency in devices.
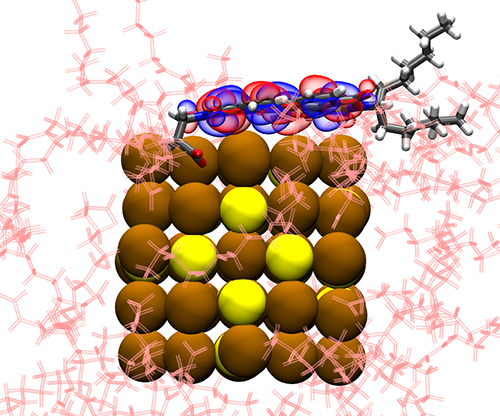
Lastly, our research on controlling charge transfer in quantum dot-dye assemblies has opened new avenues for various applications in molecular electronics, photovoltaics, and sensor technologies. In collaboration with the Roberts group at the University of Texas at Austin, the Rossky Group at Rice University, and the Gruebele group at UIUC, we investigated how organic dye molecules can regulate charge transfer between quantum dots and molecular ligands. Our computational studies revealed that increased coverage of Perylene Diimide (PDI) dye on PbS quantum dots slows down charge transfer rates due to enhanced interactions among PDI molecules. This change in interaction and conformation of the dye molecules significantly impacts electronic coupling. Our ongoing efforts involve developing machine-learned force fields for better parameterization (see my paper), aiming to accurately describe the dynamics of larger nanoparticles in solution. This research underscores the potential of functionalized quantum dots with PDI, creating versatile organic-inorganic hybrid complexes with tunable photophysical properties for diverse technological applications.